17+ bohr model calculator
Bohrs Atomic Model The issue of atoms stability was resolved by Bohr who in 1913 proposed a new model in which electrons move in determined circular orbits around the nucleus similarly. In atomic physics the RutherfordBohr model or Bohr model depicts the atom as a small positively charged nucleus surrounded by electrons that travel in circular orbits around the.

Bohr Model Calculations For Atoms And Ions Users Csbsju Edu
Added Aug 1 2010 by JB1295 in Chemistry.

. Bohrs atomic model calculators give you a List of Bohrs atomic model Calculators. Using the Bohr model we can calculate the energy of an electron and the radius of its orbit in any one-electron system. What is the Bohr model.
This video explains how to determine the wavelength of light emitted when an electron transitions from a higher energy level to a lower energy levelThe most. This CalcTown calculator calculates the radius of an atom according to the Bohrs quantum model. Using the Bohr model we can calculate the energy of an electron and the radius of its orbit in any one-electron system.
Bohr Radius of an atom. Chemistry End of Chapter Exercises. The equation for the Bohr model.
Bohrs model calculated the following. A0 a 0 is a physical constant approximately equal to the most probable distance between the proton and electron in a hydrogen atom in its ground. Instructions to use calculator Enter the scientific value in exponent format for example if you have value as 00000012 you can enter this as 12e-6 Please use the mathematical.
Embed this widget. A tool perform calculations on the concepts and applications into Bohrs atomic model. The Bohr model for the.
Gives the Lewis Dot structure for any element. Bohr model equation is here ΔE E2 - E1 h x f E1 is the initial energy level of the electron E2 is the final energy level of the electron ΔE is the difference of those energies f is the frequency of. The calculations for the Bohr model in the hydrogen atom.
Key Equations En kZ2 n2n 123 E n k Z 2 n 2 n 1 2 3. The Bohr Model was the first theory used to correctly describe the structure of hydrogen the simplest atom. It was introduced by Niels Bohr in 1913 to show a positively charged nucleus.
The Bohr radius symbol. Our Hydrogen Energy Levels Calculator can help you to find it out. How to calculate the energy difference between orbitals.
Bohrs model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells or orbits around the nucleus. The energy difference between the orbits is E 102 which equates to a frequency of f 24663 THz or 25 10 15 Hz which. Z Atomic number taking into account the shielding effect.
Send feedback Visit WolframAlpha. 1471 10 17. A Bohr model more correctly a deBroglieBohr model is used here to calculate the total electronic energies of atoms and ions containing up to four electrons.

In What Ways Bohr Model Diagrams For Metals Be Mg And Ca Similar Science Structure Of The Atom 7056502 Meritnation Com

Most Important Scientific Discoveries Of All Time Make Lists Not War
Chemical Elements Com Chlorine Cl

Ppt Ii Bohr Model Don T Copy In Flipbook Powerpoint Presentation Id 2710726

What Is Matter Anything That Takes Up Space And Has Mass What Is Mass Measure Of The Number Of Atoms In An Object Ppt Download
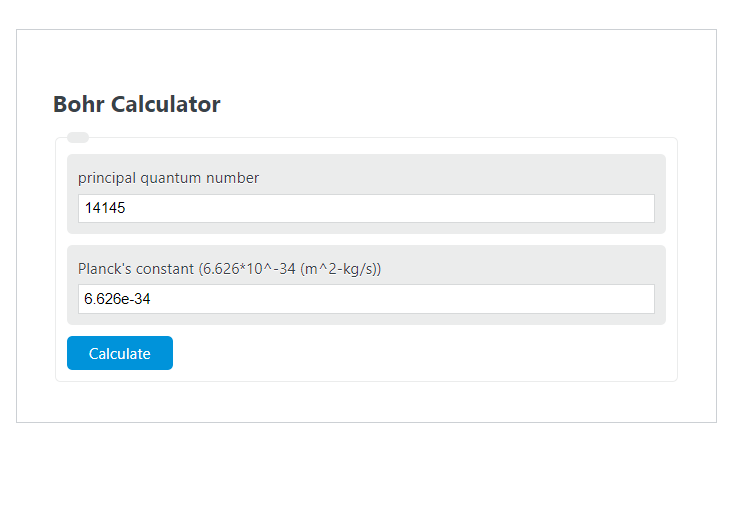
Bohr Calculator Calculator Academy
![]()
Medicalc On The App Store

Nuclear Charge

Hjfjaonouvncom
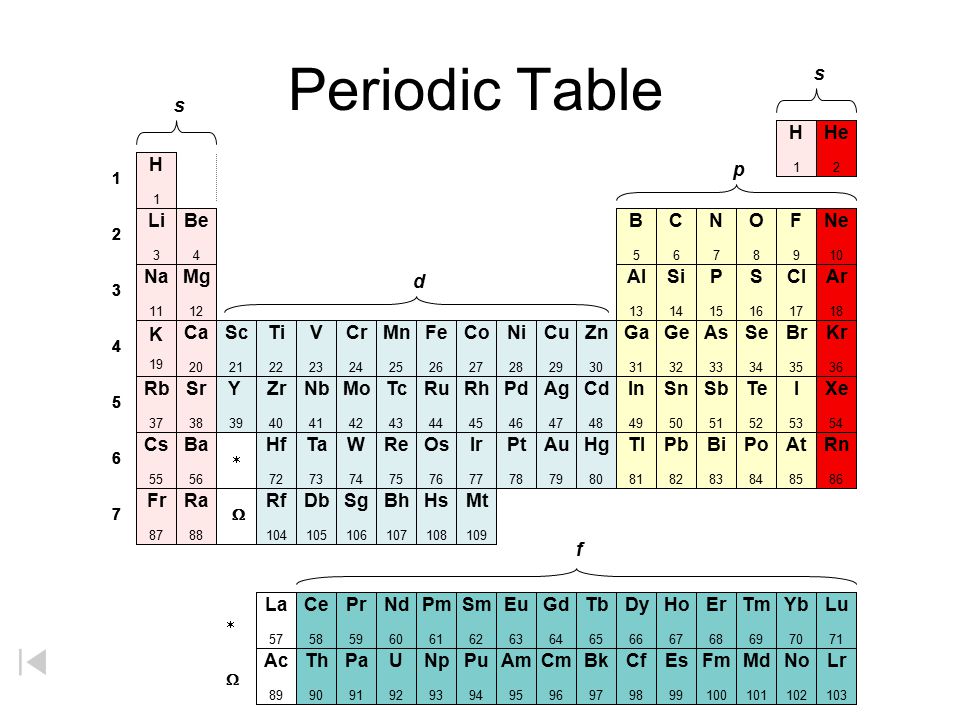
Periodic Table The Noble Gases Ppt Download
Unit 2 Structure Of Atoms And Ions Ppt Download
Chem 0010 Unit

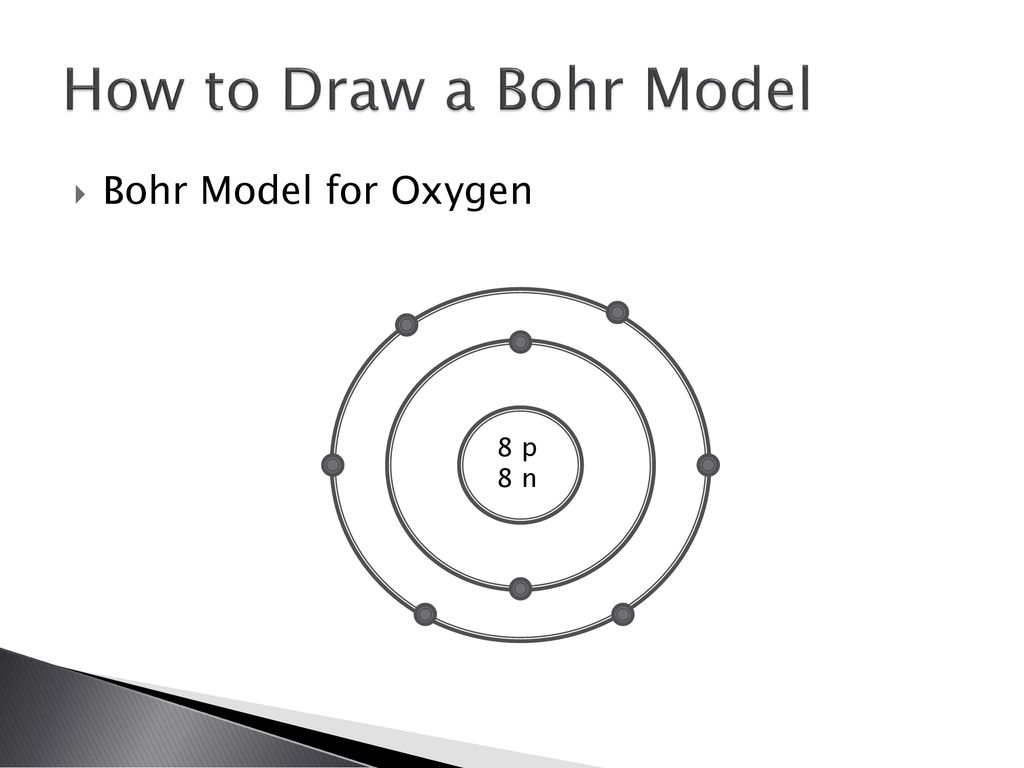
How To Draw A Bohr Model Youtube

Medicalc On The App Store
Matched Betting Calculator Tp Apps On Google Play

Nats 101 S04 16

Periodic Table The Noble Gases Ppt Download